We still report. If you are the current FDA employee or someone in the industry, with information about agency, the safety of genetic drugs or manufacturers who make them, our team wants to hear from you. Megan Rose can be reached to the signal or WhatsApp at 202-805-4865. Debbie Cemery can be reached to the signal or WhatsApp at 301-222-3133. You can also e -mail us by email (e -mail is protected).
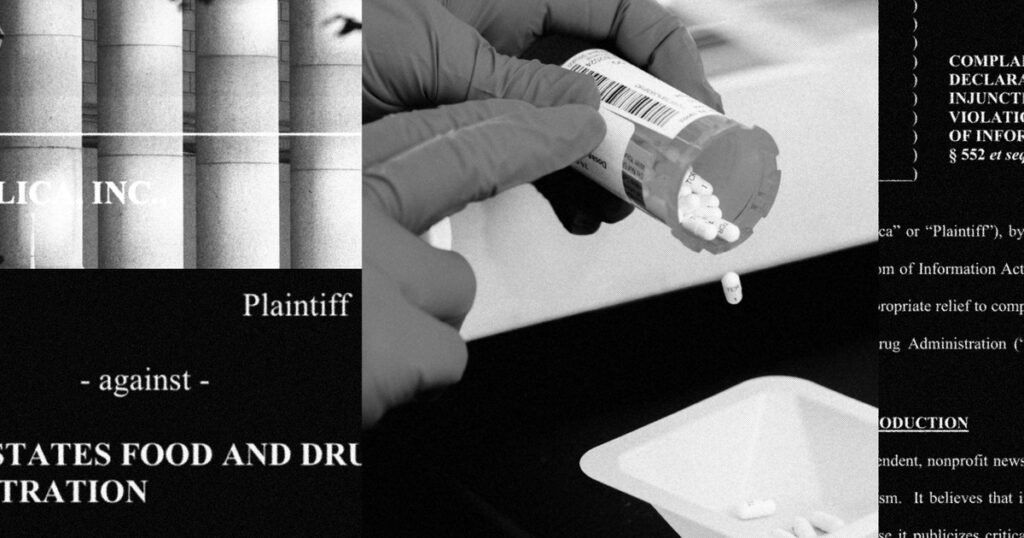
PROPUBLICA sued the US Federation and Medicines in the Federal Court in New York, accusing the agency for maintaining security information and the availability of genetic drugs that are important for millions of Americans.
Over the years, Congress, Bart, doctors and others have questioned the quality of genetic drugs made at the factories. To better understand how FDA regulates the industry and protects consumers, last year, Propublica filed four records in accordance with the Law on Freedom of Information.
The FDA refused to quickly release documents, including records that could identify drugs made at some of the most problematic plants of India. The reports on inspection describing the dangerous conditions of production are publicly available, but the FDA edits the names of medicines made at these factories.
“Americans (including pharmacists, doctors, hospital systems, politicians) do not see for themselves what drugs may have been made in dangerous objects,”, ” The lawsuit is said.
PROPUBLICA has demanded records under the permanent investigation of the total supply of drugs in America. Propublica reports The fact that FDA allowed some manufacturers to continue the delivery of drugs to Americans even after the factories that have been found in violation of quality standards and banned on the US market. More than 150 drugs or their ingredients have received these little -known exceptions over the past ten years.
In her response to the PROPBLICA request, the propublica FDA said that the information organization did not demonstrate a “convincing need” to accelerate the nomination of documents. Ever since the lawsuit was filed in November, the agency has started to transmit some requested records. The case is still acting in the federal court in New York.
PROPUBLICA claims that records will help report US consumers who are increasingly relying on common drugs made abroad. Quality problems over the years succumbed to the industry: In 2023, four people were killed after use tainted eye drops Made in India and others had to remove their eyeballs.
“Each of us relies on the FDA to ensure that the medicines we take and give our loved ones safe,” Popublica, Jack Browning, Davis Wright’s partner said. “With the increasing prevalence of maritime production, organizations such as PROPBLICA are needed to ensure that safety violations have not moved under the carpet.”
The Department of Health and Human Services, which controls the FDA, refused to comment on the case, citing the current lawsuits.
This is the second time PROPUBLICA has sued the FDA in recent years.
In 2023 in the news and Pittsburgh after the newspaper filed a lawsuit Against Agency for maintenance of records related to massive memory of breathing machines Made by Philips Resphironics. The agency eventually submitted the documents.
Daley and Nguyen are in the laboratory of the Medil North -Western University in Washington, Columbia County