Joe Demoo always knew that his healthy years could end sharply by contacting the transplanted kidney size with a small fist. But as a toddler’s father, he hoped for more time.
When he was 33, his wife sacrificed his kidney, a milestone that changed the life of the demo. Tireless fatigue, nose bleeding and itching caused by its poorly functional kidneys, and it felt good enough to leave the house in Philadelphia for a new start in the foothills of Northern California.
For a long south, the demo came to the mountains with his wife and their black and white Fasta. When his son was born, he imagined how the baseball games dressed in Phillies Gear.
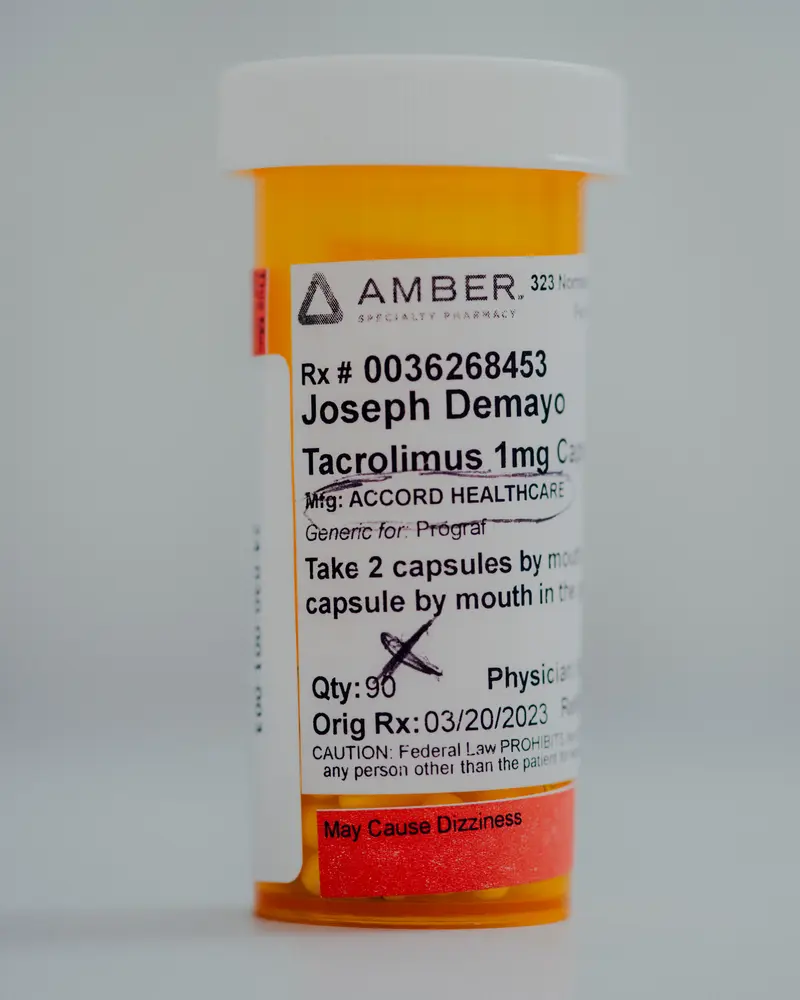
But his sacrificed kidney began to fail in the early 2023, much earlier than expected. Falling became a surprise for the demo, which in good faith took his medication, including Tserolimus immunosuppression of the drug This helps to get rid of the body.

Credit:
Using the Jo Demoo
The demo at the time did not know that the capsules he swallowed twice a day, exactly 12 hours from each other, could leave it vulnerable – or that one of the most formidable drug regulators in the world may not be able to protect it.
As it became weaker, his kidney is unable to cleanse his body with excess fluid and waste, investigators from the Power Office and medicines headed to Western India to check the plant that produced the Demoo and other common drugs for US consumers.
Since 2015, the FDA has been at least the eighth time, and each of these visits identified the problems in how the drugs were made, state records show.
During the inspection of the spring 2023. Investigators discovered that the intas pharmaceutical factory Manipulated drug testing To cover the presence of solid particles – which can include glass, fiber or other contaminants – in the company.
Without knowing the inspection, Demoo continued to accept his tco capsules. He fought with exhaustion and fought for work behind the counter.
“Dad needs a new kidney,” he recalled, telling his 5-year-old son at the time.
Credit:
George Eterge, special for propublica
In November, the FDA banned the Intas plant from drug exports to the US. But under the longtime Practice revealed to PropublicaAgency has ruled out certain medicines from the ban on the factory, including Takarolimus, which allowed drugs to continue in the United States
In a statement by Propublica, Intas whose subsidiary of the United States – Healthcare said that the company could not comment on cases of individual patients, but that its Takarolimus is safe and effective. The company said it immediately responded to the results of the FDA inspection, launching a quality focused and investing in modernization and new hires. The interest also stated that some released drugs have never been sent to the US but would not provide details.
“Intas goes on the way to the complete restoration of all production sites,” the company said.
Investigation PROPUBLICA Over the past ten years, the FDA has allowed more than 150 drugs or their ingredients from prohibited factories to the country, allegedly to prevent drug deficits.
The agency did not regularly check the medication and actively sought signs of sudden or inexplicable reactions among patients. And the liberations were largely hidden from Congress and the public, including patients such as demo who were counting on medicine to keep it alive.
The demo filled another Tacrolimus recipe just a few days before FDA released it from the Import Import Import and continued to take the capsules to the second transplant at Temple University Hospital in January 2024.
“I try to do everything right, take all my medicine,” said the 45 -year demo, which took Intas Tacrolimus for two years. “If I do it all, isn’t someone to do good care?”
In her statement, the FDA said that drug -importing manufacturers are required to carry out additional security and quality security and third party experts to assess the results before delivery to the US. The current and former FDA officials have said these measures are faulty. Many companies referred to protocols that were ineffective or prone to fraud.
The demo, which is now healed from the second transplant operation, gave the propublica two bottles of its unused Tacrolimus intas capsules. PROPUBLICA conducted their check in Valisure, Independent, accredited laboratory In Connecticut.
In their first test, Valisure scientists found that some demo pills contained a sufficient number of key ingredient, but others contained less than the minimum level set in the US Regulation. Pharmacists, doctors and other experts said insufficient They may leave patients vulnerable to the rejection of the organs.
Valisure did not find significant pollution in the demo medicine.
But scientists have found another potential problem. The capsules dissolved quickly – three times faster than the name. Experts, perhaps, rapid dissolution can introduce too much drug, potentially causing jolts, headaches and renal failure.
PROPUBLICA did not check the tutorolimus made by another manufacturer. The Intas statement states that the conclusions are “not related to inspections (FDA)” and that the FDA determined that the drug was an equivalent version of the brand when it was first approved to the US market.
Previously Valisure experienced a tutorial of interest for the Ministry of Defense Testing security and quality More than three dozen drugs are commonly used by US service members and their families. These tests also showed that the capsules dissolved too quickly.
“This is an alarming signal of other quality issues that may affect patients,” said the retired army Colonel Wick Suarez, who helped to start the Defense Department’s efforts and assist in this project.
FDA spent its Tarolimus Intas studies In recent years, they have reported a similar result on their site. The agency noted that there is no obvious risk of rejecting the organs, but said Intas Generic could create toxins in the body that can cause kidney damage. The FDA noted that capsules may not provide the same therapeutic effect as the brand version.
The conclusions have been announced In September 2023, a few weeks later, the agency predicted drugs from banning Intas imports, which allowed the company to continue delivery of Tacrolimus to the United States.
Janet Woodkok, who has been heading the FDA drug assessment and drug evaluation center for years, said in an interview that the test results were about and that the agency should quickly “try to understand”.
“It was obviously a quality problem,” she said.
Woodko did not say why the FDA released drugs from the import ban imposed on the intas factory. Despite the fact that WoodCock approved exceptions over the years, it left the center and fulfilled the duties of the main deputy commissioner of the FDA when the release from Tarolimus and other Inta drugs were made.
The demo said he never knows whether the medicine contributes to the loss of the sacrificed kidney. The refusal of the body that may occur quickly or for years is one of the most Common reasons With renal failure in patients with transplantation, but the kidneys can also for other reasons, said Joseph Vasaloti, Chief Medical Officer of the kidney National Fund.
In the case of the demo, it was hospitalized with a stomach virus and dehydration in the same year, its renal function began to decline. However, he calls into question the drug that was supposed to protect him, and worried that other patients with transplants who took over Tarolimus intas may be at risk.
A year and a half after the FDA banned drug delivery plant to the US, Tacrolimus is still expelled. The customer service agent for the company said I had recently stopped distributing drugs, but the company did not respond to a comment request.
“People who oversee the pills have failed, and people who make pills do not get,” the demo said. “How has it become so bad?”
Credit:
First and Third Photos: Hannah Yoon for Propublica. Second photo: George Ethej, special for propublica.
Lucas Waldron Contribution to schedule and development.









